What is a Diamond Heat Spreader?
A diamond heat spreader is a slim (typically ≤2 mm) sheet of synthetic (or lab-grown) diamond used to dissipate heat. It is typically placed between a heat source and a heat sink.
Diamonds aren’t normally associated with thermal management, the materials and technologies used to control heat, especially in electronic devices.
But diamonds turn out to not only be forever, but are also amazing thermal conductors used to help dissipate potentially damaging heat in specialized, high-performance electronic equipment.
Many electronic components get hot when operating, and removing that heat is crucial to component performance and longevity. Heat can change the properties of electronic components, making them less efficient and even causing damage over time. Thermal management is the science of moving heat away from components.
While diamond is universally known for its hardness, there are actually two other properties — its electrical and thermal conductivity — that are key to its fast-growing use in heat spreaders.
A brilliant conductor, better than copper or SiC
A material’s ability to move heat is measured in thermal conductivity. Thermal conductivity is measured in Watts per meter-Kelvin, W/mK. A material with a conductivity value of 1 W/mK will transfer heat at a rate of 1 watt for every degree Kelvin (or Celsius) of temperature difference across a thickness of 1 meter.
Copper is commonly used to carry away heat from electronic components. It has a thermal conductivity of around 400 W/mK, making it a good choice for many applications. Copper, however, is heavy and can easily tarnish and corrode. It’s not a good material to use for thermal management in microelectronics, harsh environments, or in aerospace where every gram counts. Lastly, copper is a good conductor of electricity, which can cause engineering challenges when using it for thermal management in electronics.
Diamond is a crystalline arrangement of common carbon. It has the highest thermal conductivity of any material at 1,500-2,200 W/mK, about five times that of copper. How does diamond conduct heat so well? The key is in its structure.
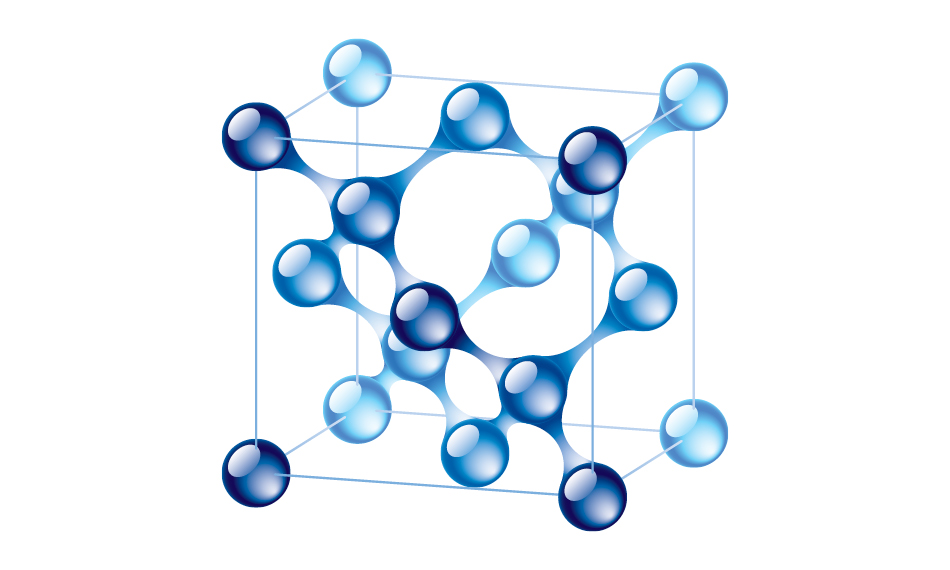
Diagram of the structure of diamond.
Diamond has a cubic crystal structure. Each carbon atom is covalently bonded to four other carbon atoms to form a tetrahedron (pyramid shape). There are no free electrons in this structure, so diamond does not conduct electricity. Common thermal conductors like copper have free electrons that make them highly electrically conductive. They can make use of peripheral electrons for heat transfer. Because diamond isn’t electrically conductive, heat is transferred only through atomic vibrations. The rigid continuous crystal structure of diamond enables these vibrations to travel very quickly through a piece of diamond. And that translates into fast heat conduction.
Purity is key to diamond’s heat conductivity. Impurities in diamond can slow down or disrupt the spread of lattice vibrations, making it less efficient at conducting heat. (Some impurities can even change the key properties of diamond. For example, natural blue diamond contains boron that makes it a semiconductor.)
Diamond’s crystal structure also make it extremely resistant to acids, bases, oxidants, and other chemicals. It’s the hardest naturally occurring material known. (Harder specialized man-made materials exist, but they don’t share diamond’s other unique properties.)
Diamond is a good material for thermal management in high-performance microelectronics. Diamond heat spreaders conduct heat much better than copper or aluminum, don’t conduct electricity, are lightweight, resistant to corrosion, and are extremely durable.
Why diamond instead of copper, aluminum or SiC
There are several reasons to use diamond as a heat conductor instead of other materials like copper, aluminum, or SiC:
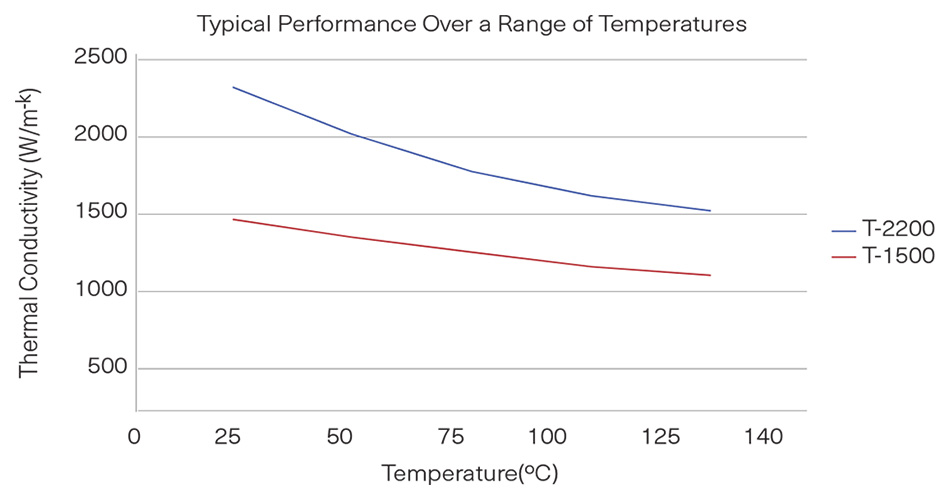
Thermal conductivity: Diamond has a thermal conductivity between 1,500-2,200 W/mK, the highest known. Copper has a thermal conductivity of 400 W/mK, aluminum 220 W/mK.
Thermal stability: Diamond doesn’t expand or contract much at different temperatures, meaning it remains very stable over a wide range of temperatures. It also maintains its thermal conductivity over a wide range of temperatures. Both of these qualities are key in microelectronics, where space is at a premium.
3. Corrosion resistance: Diamond is immune to strong acids, bases, and even organic solvents.
4. Electrical conductivity: Diamond does not conduct electricity and is a very good insulator.
5. Durability: Diamond is one of the hardest materials known.
6. Lightweight: Diamond is lightweight when compared to other common heat-conducting materials like copper.
7. Transparency: Diamond can be transparent.
How Diamond Heat Spreaders are Made
Diamond heat spreaders are made like other synthetic diamond materials. Powerful microwaves blast methane gas, releasing carbon atoms that crystalize around a seed layer of diamond. A diamond wafer grows slowly over time. Diamond heat spreaders can be made in many shapes and sizes but are typically only 2 mm or less in thickness. Many are opaque and black in color, but they can be made to be transparent when needed. Diamond heat spreaders are cut and shaped using powerful lasers or other cutting tools with diamond surfaces.
Where are Diamond Heat Spreaders Used?
Diamond heat spreaders are used anywhere engineers need highly efficient, lightweight, durable, and insulating heat conductors. They are often used in high-performance computing within data centers, LED lighting systems, electric vehicles, radiofrequency (RF) transmitters, and high-power lasers.
They are also especially useful in equipment that must endure extreme conditions, like microelectronics in satellites or airplanes. They are perfect for many aerospace uses because they are lightweight, extremely durable, electrically insulating, and can transfer heat in extreme temperatures.
Diamond heat spreaders are typically placed between heat-generating electronic components and larger passive radiators made from other materials like SiC, copper, or aluminum. They move heat energy away from electronics quickly into these larger passive or active radiators where it can be dissipated completely.
Learn more about Coherent CVD Diamond Substrates.